Neurotherapeutics Industry Breakthrough to Hit $11.10B by 2033, Key Drivers & Billion-Dollar Opportunities
“Unlocking the Brain: How Neurological Drugs and Devices Are Reshaping the Future of Neurotherapeutics in a Rapidly Expanding Global Market”
USA Neurotherapeutics Market grows from $6.97B in 2023 to $7.28B in 2024, projected $11.10B by 2033 at 4.9% CAGR.”
AUSTIN, TX, UNITED STATES, September 25, 2025 /EINPresswire.com/ -- Market Size:— DataM Intelligence 4Market Research LLP
According to DataM Intelligence, the global neurotherapeutics market size reached US$ 7.28 Billion in 2024 from US$ 6.97 Billion in 2023 and is expected to reach US$ 11.10 Billion by 2033, growing at a CAGR of 4.9% during the forecast period 2025-2033.
Get a Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):– https://www.datamintelligence.com/download-sample/neurotherapeutics-market
Key Market Highlights
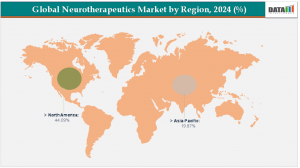
North America leads the neurotherapeutics market with a 44.1% revenue share in 2024.
Asia-Pacific is the fastest-growing region, projected to grow at a CAGR of 5.9%.
Treatment Type: Neurological drugs dominate with a 58.7% revenue share in 2024.
Major Players: Medtronic, Boston Scientific, Abbott, Aleva Neurotherapeutics, NeuroPace, Biogen, Novartis, Teva, Eli Lilly, Johnson & Johnson, and others.
The global neurotherapeutics market is undergoing a profound transformation, aptly captured by the theme “Unlocking the Brain: How Neurological Drugs and Devices Are Reshaping the Future of Neurotherapeutics in a Rapidly Expanding Global Market.” Rising neurological disease prevalence over 3 billion people worldwide living with a neurological disorder, is fueling demand for both traditional and innovative therapies. Neurological drugs dominate with established treatments like donepezil and memantine for Alzheimer’s, levodopa for Parkinson’s, and valproate and levetiracetam for epilepsy, serving as mainstays, while recent breakthroughs such as Leqembi (lecanemab) and CGRP inhibitors like Aimovig, Ajovy, and Emgality for migraine highlight the surge of novel, targeted therapies.
Meanwhile, neurological devices are emerging as the fastest-growing segment, offering precision and hope for drug-resistant patients. FDA- and CE-approved technologies such as Medtronic’s Activa DBS, Abbott’s Infinity DBS, Boston Scientific’s Vercise Genus, NeuroPace RNS for epilepsy, and non-invasive options like Neuronetics’ NeuroStar TMS and Cala Trio for essential tremor are reshaping treatment landscapes. This dual momentum, drugs offering broad accessibility and devices enabling personalized, advanced interventions, is redefining patient care. Combined with regulatory support, AI-driven personalization, and digital neurotherapeutics like EndeavorRx, the market is positioned for robust growth, making neurotherapeutics one of the most dynamic frontiers in global healthcare.
Drivers: Rising neurological disorder prevalence fuels neurotherapeutics growth, with conditions like dementia, Parkinson’s, epilepsy, and migraine affecting billions worldwide. Demand for advanced drugs, biologics, and implantable devices sustains market expansion.
Restraints: High costs of drugs and devices, such as gene therapies and deep brain stimulation, limit patient access and slow market adoption despite clinical efficacy.
Major Companies:
Major companies working towards the market's growth include Medtronic, Boston Scientific Corporation, Abbott, Aleva Neurotherapeutics, NeuroPace, Inc., Biogen, Novartis AG, Teva Pharmaceutical Industries Ltd, Eli Lilly and Company, and Johnson & Johnson, among others.
Buy Now & Unlock 360° Market Intelligence:- https://www.datamintelligence.com/buy-now-page?report=neurotherapeutics-market
Recent Developments:
• In August 2025, Eisai Co., Ltd. and Biogen Inc. announced that the U.S. Food and Drug Administration (FDA) approved the Biologics License Application (BLA) for once-weekly lecanemab-irmb subcutaneous injection LEQEMBI IQLIK for maintenance dosing. LEQEMBI IQLIK autoinjector is indicated for maintenance dosing to treat Alzheimer's disease (AD) in patients with mild cognitive impairment (MCI) or mild dementia stage of disease.
• In August 2025, Eisai Co., Ltd. and Biogen Inc. announced that the anti-amyloid beta (Aβ) monoclonal antibody LEQEMBI launched in Austria in August 2025 and in Germany in September 2025. LEQEMBI received the European Commission (EC) approval in April 2025 as the first therapy that targets an underlying cause of Alzheimer’s disease (AD). It is indicated for the treatment of adult patients with a clinical diagnosis of mild cognitive impairment (MCI) and mild dementia due to AD (collectively referred to as early AD) who are apolipoprotein E ε4 (ApoE ε4) non-carriers or heterozygotes with confirmed amyloid pathology. Germany and Austria will mark the first launches in the EU.
• In February 2025, Medtronic plc announced US Food and Drug Administration (FDA) approval of BrainSense Adaptive deep brain stimulation (aDBS) and BrainSense Electrode Identifier (EI). There is no cure for debilitating neurological conditions like Parkinson's; however, deep brain stimulation (DBS) has been transforming the lives of people with Parkinson's and other neurological disorders for more than 30 years. DBS is similar to a cardiac pacemaker, but for the brain. It uses a surgically implanted neurostimulator via a minimally invasive procedure to transmit electrical signals to specific parts of the brain affected by debilitating neurological disorders.
Market Segments
The neurological drugs segment leads the neurotherapeutics market with a 58.73% share in 2024.
In August 2025, Eisai Co., Ltd. and Biogen Inc. announced FDA approval of the Biologics License Application (BLA) for the once-weekly subcutaneous injection LEQEMBI IQLIK (lecanemab-irmb). The autoinjector is approved for maintenance treatment of Alzheimer's disease in patients with mild cognitive impairment or mild dementia.
The neurological devices segment is the fastest-growing in the neurotherapeutics market, capturing a 41.27% share in 2024.
In February 2025, Medtronic plc received FDA approval for its BrainSense Adaptive deep brain stimulation (aDBS) system and BrainSense Electrode Identifier (EI). While there is no cure for neurological disorders like Parkinson’s, deep brain stimulation (DBS) has been improving patient outcomes for over 30 years. Functioning like a pacemaker for the brain, DBS uses a surgically implanted neurostimulator to deliver electrical signals to targeted brain regions affected by debilitating neurological conditions.
North America Neurotherapeutics Market
North America is set to lead the global neurotherapeutics market with a 44.09% share in 2024, driven by robust healthcare infrastructure, advanced R&D, and supportive regulatory and reimbursement frameworks. Strong venture capital investments, a specialized workforce, and active patient advocacy further accelerate commercialization and adoption of innovative therapies.
US Market Trends
The US faces a high neurological disease burden, with over 6 million Alzheimer’s patients, 1+ million Parkinson’s cases, and 3.4 million people with epilepsy. FDA approvals for breakthrough drugs like Leqembi and CGRP inhibitors (Aimovig, Ajovy, Emgality) have fueled rapid uptake. On the device side, Medtronic’s Activa DBS, Abbott’s Infinity DBS, Boston Scientific’s Vercise Genus, NeuroPace RNS, and Neuronetics’ NeuroStar TMS demonstrate leadership in both invasive and non-invasive neuromodulation therapies, alongside FDA-cleared wearables like Cala Trio and gammaCore.
Asia Pacific Market Trends
Asia Pacific is the fastest-growing region with a 5.9% CAGR in 2024, driven by a large patient population, rising healthcare investments, and improved access to advanced therapies. China and India together account for over 300 million people aged 60+, driving Alzheimer’s, Parkinson’s, and stroke prevalence. Increasing awareness and government support are boosting diagnosis and treatment adoption. Multinational drugs like donepezil, memantine, levodopa, and recent CGRP inhibitors are entering the market, while advanced neuromodulation devices are seeing growing adoption supported by expanding surgical expertise.
Europe Market Trends
Europe shows strong growth due to an aging population, high neurological disease prevalence, and supportive regulatory frameworks. Over 10 million people live with dementia and 1.2 million with Parkinson’s. EMA and EC approvals for key drugs such as donepezil, rivastigmine, memantine, levodopa, safinamide, and rasagiline sustain the market. In August–September 2025, Eisai and Biogen launched LEQEMBI, the first anti-amyloid beta therapy targeting Alzheimer’s disease, beginning in Austria and Germany, following EC approval in April 2025 for patients with early-stage AD.
Get Customization in the report as per your requirements:- https://www.datamintelligence.com/customize/neurotherapeutics-market
Related Reports
Neurology Devices Market
Interventional Neurology Devices Market
About Us:
DataM Intelligence 4Market Research is a market intelligence platform that gives access to syndicated, customized reports and consulting to its clients in one place. As a firm with rich experience in research and consulting across multiple domains, we are a one-stop solution that will cater to the needs of clients in key business areas. DataM Intelligence has an online platform whose coverage includes industries such as chemicals and materials, agriculture, health care services, animal feed, and food & beverages among others.
Our platform has Insights on markets that uncover the latest market research data that are distinct from the competition. With coverage across 10 major industries in the marketplace research, DataM Intelligence benefits thousands of companies by helping them take their innovations early to the market, and by providing a complete view of the market with statistical forecasts. Our strategy-centric framework and value-added services will let individuals and corporates with ease of access and custom personalization to research and markets.
Sai Kiran
DataM Intelligence 4Market Research LLP
877-441-4866
sai.k@datamintelligence.com
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.